Car T Therapy Canada
CAR-T therapy is still considered a new and innovative therapy for the treatment of lymphoma. Y B-Cell Acute Lymphoblastic Leukemia B-cell ALL in patients ages 3-25.
KYMRIAH tisagenlecleucel and YESCARTA axicabtagene ciloleucel.
. Health Canada approved Kymriah tisagenlecleucel in September. CAR T-cell therapynew hope when standard cancer treatments fail. CAR T Cell Therapy.
However she was eligible for a novel immunotherapy clinical trial using CAR T-cells which ultimately saved her life. Ad From Biomarker Identificaiton to Clinical Trial. Availability of CAR T-cell Therapy in Canada.
Learn More About CAR T Treatment For Relapsed Multiple Myeloma. CAR T-cell therapy is a precision medicine treatment meaning treatment that is tailored to individual patients. However pricing and reimbursement in Canada is ongoing.
23 To date there has been no intellectual property litigation related to either of. Y Diffuse Large B-cell Lymphoma DLBCL in patients 18 and older after. Chimeric antigen receptor CAR T-cell therapy is a way to get immune cells called T cells a type of white blood cell to fight cancer by changing them in the lab so they can find and destroy cancer cells.
CAR T-cell therapy is also sometimes talked about as a type of cell-based gene therapy because it involves altering the genes inside T cells to help them attack the cancer. Health care system planners must consider the opportunity costs that arise in adopting CAR T-cells beyond their current indications. Challenges for Implementation in Canada.
It also belongs to a new group of cancer treatments known as immunotherapy. Chimeric antigen receptor CAR T-cell therapy is a rapidly emerging and novel therapy which utilizes a patients own immune cells to treat their cancer. Provide High-Quality Custom Service Covering The Entire CAR-T Therapy Development Process.
Its a form of CAR-T immunotherapy in which a patients blood cells are removed reprogrammed to attack cancer and then re-injected. They may even continue to grow and work over time. Before the development of CAR T cells many of these patients were virtually untreatable.
A study co-led by Dr. Natasha Kekre at The Ottawa Hospital is investigating the use of specialized chimeric antigen receptor T CAR-T cells to. A type of white blood cell called a.
When Stefany Duponts leukemia returned after her bone marrow transplant the prognosis was dire. As CAR T-cell therapy has become more widely available Dr. Amanda Li MD MSc FRCPC Clinical Assistant Professor UBC Pediatric Hematology Oncology BMT hildrens Hospital 1.
The innovation they represent demanded reciprocal innovation. The Ottawa Hospital is developing a made-in-Canada CART -cell clinical trial to help other. CD19-targeted CAR T cells are also offering hope to adults and children with advanced aggressive lymphomas.
They represent a unique approach to treatment that challenged Canadas standard health technology assessment HTA and reimbursement pathways. CAR-T therapy has received approval from Health Canada for limited oncology indications. One-Stop Solution CAR-T Service.
In 2018 regulatory approval in the UK and Canada followed suit approving. CAR T-cell therapy is called a living therapy That is because the CAR T-cells go on to multiply in the body and continue fighting the cancer. While Ontario is building capacity for CAR T-cell therapy the province can now treat a limited number of patients from Ontario and other provinces and territories.
He first two chimeric antigen receptor T-cell CAR-T therapies were approved for the Canadian market in September 2018 Kymriah and February 2019 Yescarta. In Canada CAR T therapy is currently regulated in the same way as pharmaceuticals and there are currently two commercial CAR T therapies that have been approved for use in Canada. Ontario hospitals are funded to deliver CAR T-cell therapy for patients who meet the eligibility criteria.
In Canada CAR T therapy is currently regulated in the same way as pharmaceuticals and there are currently two commercial CAR T therapies that have been approved for use in Canada. In CAR T a persons T-cellswhich are among the bodys most important immune cellsare taken from their blood and then infused into the persons bloodstream. Vulnerable families may mortgage the future for the promise of a cure that is imperfect.
Many just wont have the ability to finance this therapy. CAR T-cell therapy involves taking the T-cells from a patient genetically modifying them to fight cancer cells expanding their numbers in a. CAR T Cells Explained Family Practice Oncology Network CME Day November 23 2019 Dr.
CAR T therapy starts by extracting a patients immune cells from their body. This is why patients typically need only one CAR T-cell infusion. Fry said it has rapidly become the standard of care for children with relapsed ALL.
Researchers are developing systems that could put Canada on the map for adoptive cell therapy for leukemia and other conditions. Chimeric Antigen Receptor modified T cells known as CAR T is a powerful new tool for treating cancer. The cells are genetically engineered to recognize a patients own tumour and then returned to the patients body in large numbers.
These types of new CAR therapies are enormously expensive. It was initially approved by Health Canada in 2018 for relapsedrefractory Diffuse Large B-Cell Lymphoma patients and more recently in 2021 for Mantle Cell Lymphoma however it is still not locally accessible for all patients across Canada. In 2017 autologous chimeric antigen receptor CAR T-cell therapy tisagenlecleucel Kymriah Novartis became the first gene therapy approved in the United States for acute lymphoblastic leukemia in children and young adults.
CAR T-cell therapy is a type of gene therapy that trains or engineers a patients own immune system to recognize cancerous cells. The process to produce and deliver CAR T-cell therapy is complex. Treatment of some blood cancers including.
Ad FDA Approved CAR T RR MM Treatment Option For Patients. Visit the Canadian Cancer Society to watch a video about how CAR T-cell therapy works. Kevin Hay from Vancouver Coastal Health Research Institute VCHRI and Dr.
CAR T-cell therapies have been approved in Canada as the standard of care for.

Global Manufacturing Of Car T Cell Therapy Molecular Therapy Methods Clinical Development
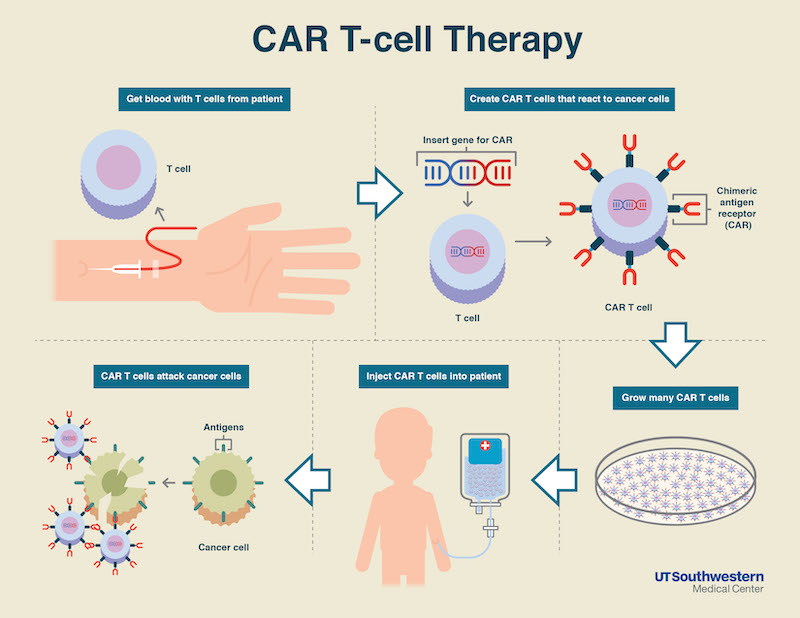
New Car T Cell Therapy Extends Remission In Heavily Relapsed Multiple Myeloma Patients Newsroom Ut Southwestern Dallas Texas
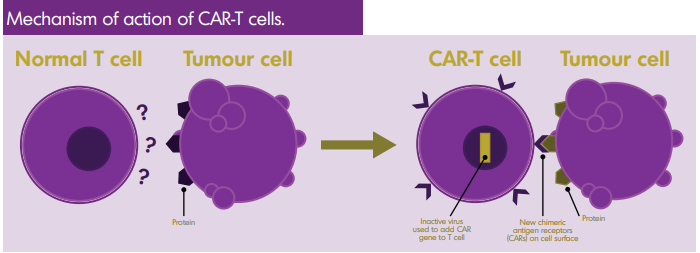
No comments for "Car T Therapy Canada"
Post a Comment